Abstract
Background
Although anthracyclines are considered as being among the most potent chemotherapeutic agents for endometrial carcinoma, the majority of institutions in Japan prefer a combination of paclitaxel and carboplatin (TC) for treating this disease. We retrospectively evaluated the efficacy and feasibility of combined paclitaxel, pirarubicin, and carboplatin (TPC) therapy for endometrial carcinoma.
Methods
Thirty-nine patients with high/intermediate postoperative recurrence risks or with advanced disease received combination chemotherapy consisting of paclitaxel (150 mg/m2), pirarubicin (35 mg/m2), and carboplatin [area under the concentration time curve (AUC = 4)] from 2001 to 2006 at Okayama University Hospital. Treatment cycles were repeated every 3 weeks, and three to nine cycles were administered according to patient risk.
Results
The 1-year overall survival (OS) and progressionfree survival (PFS) rates were 94.9% and 84.6%, respectively, and the 3-year OS and PFS rate was 81.3%. Hematologic toxicities [grade 3 were: anemia 30.8%; leukopenia 84.6%; thrombocytopenia 20.5%. Neutropenia was common, and administration of granulocyte colonystimulating factor (G-CSF) was necessary in 87.9% of treatment courses. Although grade 3 or 4 neutropenia was unavoidable, we could administer TPC therapy safely and without delay with G-CSF support. Gastrointestinal and neurological toxicity were less severe and less frequent compared with TC, and no cardiac toxicity was observed.Conclusion The 3-year PFS and OS rates even in highrisk patients were satisfactory, and we confirmed the feasibility of using this regimen for treating endometrial carcinoma.
Keywords Endometrial cancer . TPC therapy
Introduction
Anthracyclines are considered as being among the most potent chemotherapeutic agents for endometrial carcinoma and have been used as key drugs for treating this disease.Previous studies have reported the efficacy of doxorubicin and cisplatin (AP) [1–3] and of cisplatin, doxorubicin, and cyclophosphamide (CAP) [4–7] for advanced or recurrent endometrial carcinoma. Several randomized studies have been reported the superiority of AP over doxorubicin alone [8, 9] and whole-abdominal irradiation [10]. A randomized trial comparing AP and doxorubicin plus paclitaxel (AT) revealed no difference [11]. Then, Fleming et al. [12, 13] conducted a dose-escalating study of paclitaxel with fixed doses of cisplatin and doxorubicin (TAP), and a randomized study comparing AP and TAP was conducted. TAP yielded a significantly better response rate (RR), progression-free (PFS), and overall (OS) survival than AP but was also associated with a high risk of peripheral neuropathy. A randomized study comparing TAP with paclitaxel and carboplatin (TC) is ongoing. In Japan, the guideline of the Japan Society for Gynecologic Oncology recommends combination chemotherapy with an anthracycline and a platinum agent for treating endometrial carcinoma. However, many institutions in Japan still prefer TC without anthracyclines. In this study, we retrospectively evaluated the potency and feasibility of TPC chemotherapy in patients with endometrial carcinoma.
Patients and methods
Patient criteria
Between 1 January 2001 and 31 December 2006, 39 patients with endometrial carcinoma were treated with TPC at Okayama University Hospital. The patients provided written informed consent to this treatment. Of the 39 patients, 33 were postoperative patients without measurable residual lesions but had a histologically confirmed high or intermediate recurrence risk [14, 15] and received TPC therapy in an adjuvant setting; the remaining six patients had advanced disease and received treatment as neoadjuvant chemotherapy. Patients were required to have no myelosuppression (granulocyte count\1500/ll, platelet count\15000/ll), renal dysfunction (creatinine[1.5 times the upper limit), or hepatic dysfunction (aminotransferase [2.5 times the upper limit of normal). Patients with a past or present history of heart failure, Eastern Cooperative Oncology Group (ECOG) performance status[2, and any histotypes of sarcoma were excluded. Surgical staging was performed at the time of the initial operation or before the first cycle of neoadjuvant chemotherapy according to the International Federation of Gynecology and Obstetrics (FIGO) criteria established in 1988.
Chemotherapy regimen
Paclitaxel (150 mg/m2), pirarubicin (35 mg/m2), and carboplatin [area under the concentration time curve (AUC = 4)] were administered by intravenous infusion on the same day, with pirarubicin first over 30 min, followed by paclitaxel over 3 h, and then carboplatin over 1 h. Treatments were repeated every 21 days. Patients with intermediate recurrence risk, such as grade 3 endometrioid adenocarcinoma and type II carcinomas, grade 1 or 2 endometrioid adenocarcinoma with myometrial invasion to more than one half of the endometrium, cervical invasion,or lymph–vascular space involvement, received three courses of TPC therapy. Patients wtih high recurrence risk,such as, uterine-serosal or adnexal involvement, vaginal involvement, lymph node metastasis, intra-abdominal dissemination, or distant metastasis, received six courses of TPC therapy. Three postoperative patients with omental metastasis and/or peritoneal dissemination received nine courses of adjuvant TPC therapy. Prophylactic 5-hydroxytryptamine (5-HT3) receptor antagonists and other antiemetic treatments were given to all patients.
Granulocyte colony-stimulating factor (G-CSF) was administered strictly according to the Japanese modification (by the Japanese Society for Clinical Oncology) of the American Society for Clinical Oncology (ASCO) guidelines.
Evaluation
PFS and OS were estimated using the Kaplan–Meier method. Toxic events and treatment-related complications were evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) ver. 3.0. The clinical response in patients receiving treatment as neoadjuvant chemotherapy was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1.
Results
Patient characteristics
Characteristics of the patients are listed in Table 1. The median age was 56 (range 33–69) years. ECOG performance status was 0 in 36 patients and one in three patients. Surgical stage was Ib in five patients, Ic in five, IIa in three,
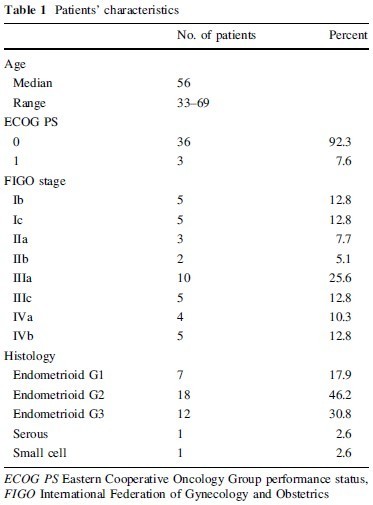
IIb in two, IIIa in ten, IIIc in five, IVa in four, and IVb in five. Three stage IVb patients, one stage IVa patient with rectal invasion, and two stage III patients with intrapelvic dissemination and bulky ovarian metastasis received TPC as neoadjuvant chemotherapy. The histological diagnosis was endometrioid adenocarcinoma grade I in seven patients, grade II in 18, and grade III in 12, and serous adenoc arcinoma in one and small cell carcinoma in one.
Treatment
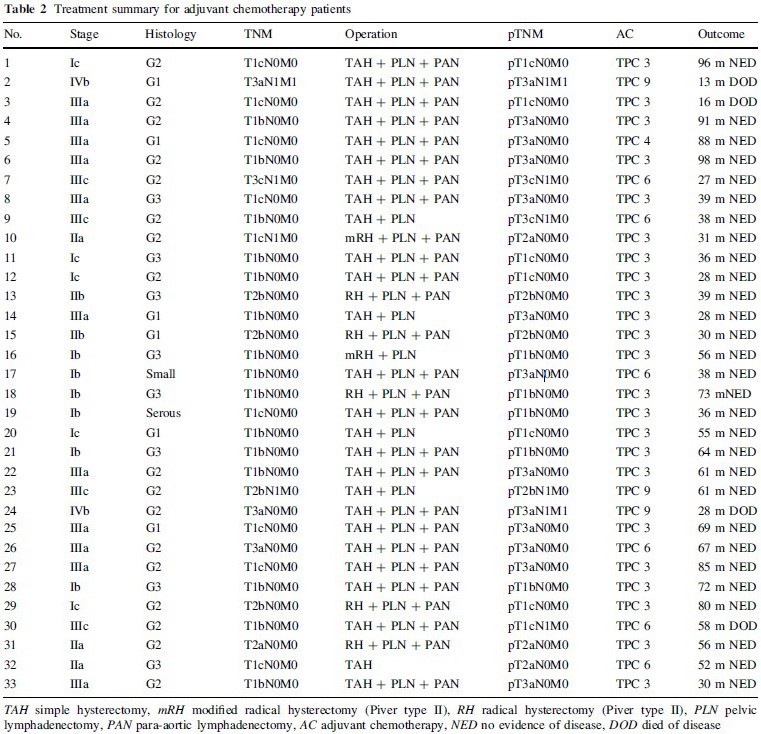
Surgical and chemotherapeutic treatments in the 33 patients who received TPC as adjuvant chemotherapy are summarized in Table 2. Twenty-three patients with a moderate postoperative risk of recurrence received three courses of TPC, and one received four courses. Six patients with a high postoperative risk of recurrence received six courses of TPC, and three received nine courses. Six advanced endometrial carcinoma patients received three to five courses of TPC as neoadjuvant chemotherapy, followed by the initial surgery (Table 3).
All 33 patients, excluding the six who received TPC as neoadjuvant chemotherapy, underwent at least simple abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) as the initial operation. In addition, four patients underwent pelvic lymph node
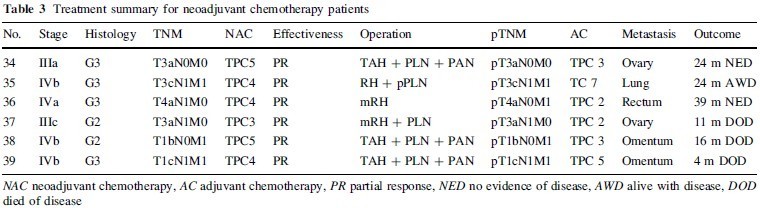
dissection (PLN) and partial omentectomy (pOMT), 21 underwent PLN and para-aortic lymph node dissection (PAN) with total omentectomy (OMT), two underwent semiradical hysterectomy (Piver type II: mRH) because of suspected involvement of the cervix, and five with frank cervical involvement underwent radical hysterectomy (Piver type III: RH) with PLN, PAN, and OMT. The average numbers of dissected lymphnodes were 38.6 for PLN and 25.1 for PAN. One stage IVb patient with lung metastasis underwent RH with PLN sampling and pOMT due to parametrial involvement after four courses of TPC. Two stage IVb patients with massive omental metastases underwent TAH with PLN, PAN, and OMT after four or five courses of TPC. One stage IVa patient underwent mRH and partial resection of the rectum after four courses of TPC. One stage IIIc patient with bulky ovarian metastasis and cervical involvement underwent mRH, PLN, and pOMT after three courses of TPC. One state IIIa patient with bulky ovarian metastasis underwent TAH, PLN, and PAN after five courses of TPC.
Survival and response rates
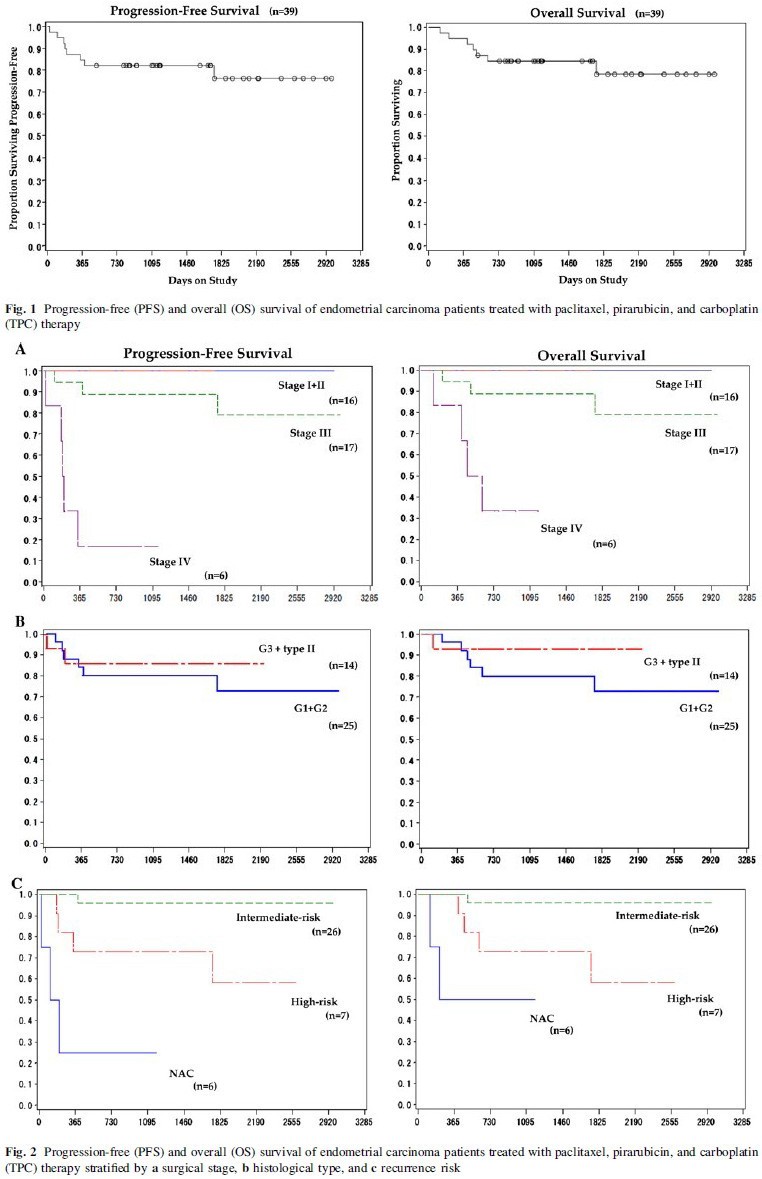
Figure 1 shows PFS and OS of the 39 patients who received TPC therapy. The median follow-up period was 39 (range 4–98) months. The 1-year OS and PFS rates were 94.9% and 84.6%, respectively, and the 3-year OS as well as PFS rate was 81.3%. Figure 2 shows survival curves according to stage, grade, histotype, and postoperative recurrence risk. Although the number of patients was small, the 3-year OS and PFS rates for high-risk patients were both 71.4%. Among the six patients who received TPC therapy as neoadjuvant chemotherapy, tumor response was classified according to RECIST ver. 1.1. Although the patient number was small, OS rate was 100% [partial response (PR) in six patients]. Although all patients retained some viable cells at the time of initial surgery after neoadjuvant chemotherapy, two patients attained >70% tumor reduction.
Toxicity and feasibility
Adverse events are summarized in Table 4. Hematologic toxicities>grade 3 were anemia 30.8%; leukopenia 84.6%; neutropenia 100%; thrombocytopenia 20.5%. Neutropenia was unavoidable in all cases, and administration of G-CSF was necessitated in 87.9% of treatment courses. There were no cases of febrile neutropenia. The average treatment interval was 23.4 days, and the longest interval was 34 days. In four patients (total nine courses), paclitaxel dose needed to be reduced from 150 to 135 mg/m2 due to prolonged myelosuppression. However, the intended treatment could be completed as intended in all 39 patients with G-CSF support.
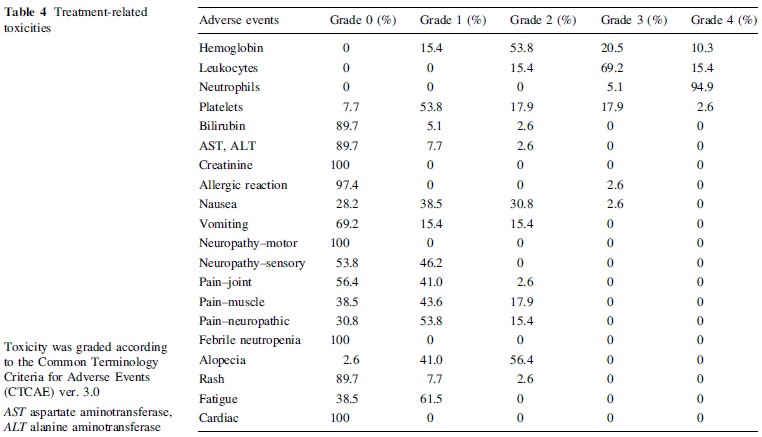
Nonhematologic toxicities [grade 3 were very rare. Most gastrointestinal events were no greater than grade 2. Neuralgia\grade 3 was common (grade 1 in 21 patients;grade 2 in six patients). Alopecia was relatively common,and grade 2 alopecia was found in 22 patients (56.2%). No cardiac adverse events were observed.
Discussion
Treatment guidelines of the Japan Society for Gynecologic Oncology recommend combination chemotherapy with an anthracycline and a platinum agent for treating endometrial carcinoma. Nevertheless, most Japanese institutions prefer TC without an anthracycline. This may be mainly because we are accustomed to using TC, especially for treating ovarian carcinoma. Moreover, we may hesitate to use doxorubicin because of the result of a meta-analysis study showing the absence of any survival benefit and a higher incidence of adverse events with doxorubicin with CAP compared with CP for ovarian carcinoma [16]. Previously, Hidaka et al. [17] showed a favorable outcome of endometrial carcinoma in patients treated with TC rather than with CAP, and there are several reports of high RRs of advanced and recurrent endometrial carcinoma to TC [18– 20]. However, a phase III trial by the Gynecological Oncology Group (GOG) comparing AP and TAP [13] clearly demonstrated favorable RR, PFS, and OS rates in patients treated with TAP. A phase II study of paclitaxel, epirubicin, and carboplatin (TEC) for advanced and recurrent endometrial carcinoma revealed a favorable RR of 63.2% [21]. Results of a meta-analysis by Humber et al. [22]. also indicated a high efficacy of a platinum drug, anthracycline, and paclitaxel in patients with advanced or recurrent endometrial carcinoma.
The problems associated with the use of TAP are the high incidence of peripheral neuropathy (grade 2 and 3 in 39%) and myelosuppression (grade 2–4 in 71%) [13]. Also, grade II–III cardiac toxicity was found in 12% of patients [13]. In order to improve treatment compliance and overcome the high incidence of cardiac toxicity of TAP, we selected pirarubicin, a synthetic analogue of doxorubicin. The maximum dose of pirarubicin allowed (950 mg/m2) is greater than that of epirubicin (900 mg/m2) or doxorubicin (500 mg/m2). We also selected carboplatin to reduce the incidence of gastrointestinal and renal toxicities and peripheral neuropathy. We fixed the dose of pirarubicin at 35 mg/m2. We started a preliminary study with 180 mg/m2 paclitaxel and carboplatin AUC = 5 but could not continue with these doses because of prolonged hematologic toxicities. Therefore, we reduced paclitaxel to 150 mg/m2 and carboplatin to AUC = 4. With these reduced doses, we could administer TPC safely with G-CSF support. These doses are exactly the same as those used in a Japanese multicenter, phase I, dose-escalation study conducted by Sugiyama et al. (not published). Fleming et al. [13] routinely used prophylactic G-CSF administration from day 3 to day 12 in patients treated with TAP, lending support to this practice. Similarly, severe hematologic toxicities, especially grade III or IV neutropenia, were almost unavoidable in our study. However, we could administer TPC therapy safely, without delay, with G-CSF support. Neuromuscular toxicities, including myalgia, arthralgia, and peripheral nerve pain were less severe and less frequent than in our ovarian cancer cases treated with TC and in other reports [17–20] and also significantly less frequent than those observed with TAP [13]. This may be because we used 150 mg/m2 instead of 180 mg/m2 paclitaxel in TC or 160 mg/m2 paclitaxel in TAP. Carboplatin might be associated with a lower incidence of peripheral neuropathy than that of cisplatin in TAP. Gastrointestinal events were relatively less severe and less frequent, and no cardiac toxicities were observed in this study.
We confirmed the feasibility of using the combination regimen composed of a reduced dose of paclitaxel, pirarubicin as the anthracycline, and carboplatin for treating endometrial carcinoma. Although the number of patients in this study was small, the 3-year PFS and OS rates were satisfactory, even in patients with a high recurrence risk and those with high-grade endometrioid adenocarcinoma and type II histotypes (Fig. 2). We could assess the response of TPC therapy in only six advanced patients, and only seven high-risk patients were treated with adjuvant TPC therapy. Further investigation is needed exclusively for these advanced and high-risk endometrial patients to clarify the potency of TPC therapy. Recently, results of a phase III randomized trial of AP or TAP after optimal surgery plus volume-directed radiation in patients with advanced endometrial carcinoma were published [23]. There was little difference in the recurrence-free period between patients treated with AP and TAP. However, in a subgroup analysis, TAP was found to be associated with a significantly reduced risk of recurrence or death among patients with gross residual disease. Combination chemotherapy with taxane, anthracycline, and a platinum agent could yield high RRs, especially in the small population of endometrial carcinoma patients with unfavorable prognosis. However, caution must be exercised while selecting the appropriate candidates for this therapy and in dealing with the high frequency of adverse events.
Conflict of interest statement No author has any potential conflict of interest.
References
1. Trope´ C, Johnsson JE, Simonsen E et al (1984) Treatment of recurrent endometrial adenocarcinoma with a combination of doxorubicin and cisplatin. Am J Obstet Gynecol 149:379–381
2. Pasmantier MW, Coleman M, Silver RT et al (1984) Treatment of advanced endometrial carcinoma with doxorubicin and cisplatin: effects on both untreated and previously treated patients. Cancer Treat Rep 69:539–542
3. Seltzer V, Vogt SE, Kaplan BH (1984) Adriamycin and cis-diamminedichloroplatinum in the treatment of metastatic endometrial adenocarcinoma. Gynecol Oncol 19:308–313
4. Turbow MM, BAllon SC, Sikic BI et al (1985) Cisplatin, doxorubicin, and cyclophosphamide chemotherapy for advanced endometrial carcinoma. Cancer Treat Rep 69:465–467
5. Edmonson JH, Krook JE, Hilton JF et al (1987) Randomized phase II studies of cisplatin and a combination of cyclophosphamide- doxorubicin-cisplatin (CAP) in patients with progestinrefractory advanced endometrial carcinoma. Gynecol Oncol 28:20–24
6. Burke TW, Stringer CA, Morris M et al (1991) Prospective treatment of advanced or recurrent endometrial carcinoma with cisplatin, doxorubicin, and cyclophosphamide. Gynecol Oncol 40:264–267
7. Dunton CJ, Pfeifer SM, Braitman LE et al (1991) Treatment of advanced and recurrent endometrial cancer with cisplatin, doxorubicin, and cyclophosphamide. Gynecol Oncol 41:113–116
8. Thigpen JT, Brady MF, Homesley HD et al (2004) Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 22:3902–3908
9. Aapro MS, van Wijk FH, Bolis G et al (2003) Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomized study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol 14:441–448
10. Randall ME, Filiaci VL, Muss H et al (2006) Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 24:36–44
11. Fleming GF, Filiaci VL, Bentley RC et al (2004) Phase III randomized trial of doxorubicin ? cisplatin versus doxorubicin ? 24-h paclitaxel ? filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol 15:1173–1178
12. Fleming GF, Fowler JM, Waggoner SE et al (2001) Phase I trial of escalating doses of paclitaxel combined with fixed doses of cisplatin and doxorubicin in advanced endometrial cancer and other gynecologic malignancies: a Gynecologic Oncology Group study. J Clin Oncol 19:1021–1029
13. Fleming GF, Brunetto VL, Cella D et al (2004) Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 22:2159–2166
14. Boronow RC, Morrow CP, Creasman WT et al (1984) Surgical staging in endometrial cancer: clinical-pathological findings of a prospective study. Obstet Gynaecol 63:825–835
15. Morrow CP, Bundy BN, KUrman RJ et al (1991) Relationship between surgical–pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40:55–65
16. West RJ, Zweig SF (1997) Meta-analysis of chemotherapy regimens for ovarian carcinoma: a reassessment of cisplatin, cyclophosphamide and doxorubicin versus cisplatin and cyclophosphamide. Eur J Gynaecol Oncol 18:343–348
17. Hidaka T, Nakamura T, Shima T et al (2006) Paclitaxel/carboplatin versus cyclophosphamide/adriamycin/cisplatin as postoperative adjuvant chemotherapy for advanced endometrial adenocarcinoma. J Obstet Gynaecol Res 32:330–337
18. Hoskins PJ, Swenerton KD, Pike JA et al (2001) Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol 19:4048–4053
19. Michener CM, Peterson G, Kulp B et al (2005) Carboplatin plus paclitaxel in the treatment of advanced or recurrent endometrial carcinoma. J Cancer Res Clin Oncol 131:581–584
20. Akram T, Maseelall P, Fanning J (2005) Carboplatin and paclitaxel for the treatment of advanced or recurrent endometrial cancer. Am J Obstet Gynecol 192:1365–1367
21. Papadimitriou CA, Bafaloukos D, Bozas G et al (2008) Paclitaxel, epirubicin, and carboplatin in advanced or recurrent endometrial carcinoma: a Hellenic Co-operative Oncology Group (HeCOG) study. Gynecol Oncol 110:87–92
22. Humber CE, Tierney JF, Symonds RP et al (2007) Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Ann Oncol 18:409–420
23. Homesley HD, Filiaci V, Gibbons SK et al (2009) A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol 112:543–552

